Abstract
Background: Caffeine is commonly used as therapy for apnea of prematurity and has shown potential in preventing other conditions in preterm neonates. However, the optimal timing for caffeine therapy remains uncertain. Objective: This study aimed to compare the outcomes of early versus late administration of caffeine in preterm neonates. Methods: PubMed, Embase, and Cochrane Library were searched for studies comparing 0–2 days to ≥3 days caffeine introduction in preterm neonates. Outcomes included were mortality, bronchopulmonary dysplasia (BPD), intraventricular hemorrhage (IVH), necrotizing enterocolitis (NEC), retinopathy of prematurity (ROP), patent ductus arteriosus (PDA), late-onset sepsis, length of hospital stay, and the composite of BPD or death. RevMan 5.4.1 was used for statistical analysis. Results: A total of 122,579 patients from 11 studies were included, 2 were randomized controlled trials (RCTs), and 63.9% of the neonates received early caffeine administration. The rates of BPD (OR: 0.70; 95% CI: [0.60–0.81]; p < 0.0001), IVH (OR: 0.86; 95% CI: [0.82–0.90]; p < 0.0001), ROP (OR: 0.80; 95% CI: [0.74–0.86]; p < 0.0001), late-onset sepsis (OR: 0.84; 95% CI: [0.79–0.89]; p < 0.00001), and PDA (OR: 0.60; 95% CI: [0.47–0.78]; p < 0.0001) were significantly reduced in the early caffeine group. The composite outcome of BPD or death was also lower in the early caffeine group (OR: 0.76; 95% CI: [0.66–0.88]; p < 0.0003). Mortality rate was higher in the early caffeine group (OR: 1.20; 95% CI: 1.12–1.29; p < 0.001). Conclusion: As compared with late caffeine administration, early caffeine is associated with a reduction in BPD, IVH, ROP, late-onset sepsis, and PDA in preterm neonates, albeit increased mortality. Additional RCTs are warranted to confirm these findings and evaluate whether the effect on mortality may be related to survival bias in observational studies favoring the late treatment group.
Introduction
Methylxanthines are frequently prescribed medications for treating apnea of prematurity in neonates, with caffeine currently being the most utilized agent in this category. “Caffeine for Apnea of Prematurity” is a randomized clinical trial published in 2006 that has significantly influenced the use of caffeine in premature newborns since its publication [1]. Additionally, a secondary analysis of this trial demonstrated that initiating caffeine treatment within the first 3 days of life was associated with respiratory benefits. Infants who were administered caffeine had a lower occurrence of bronchopulmonary dysplasia (BPD) and a shorter duration of mechanical ventilation compared to the control group.
BPD is a prevalent respiratory complication among preterm infants, affecting approximately 42% of neonates born before 28 weeks of gestation [2]. Caffeine is extensively employed in the treatment and prevention of such complications during the neonatal period [3]. However, despite the existence of evidence, there are currently no consistent data indicating the optimal timing for initiating caffeine therapy after birth. Consequently, many institutions have not yet implemented early caffeine administration as a standard therapeutic approach.
Several systematic reviews and two meta-analyses have been previously conducted, with the most recent one published in 2017 and incorporating articles published prior to 2016 [4, 5]. Consequently, our objective was to conduct an updated systematic review and meta-analysis to examine the optimal timing for initiating caffeine therapy in preterm newborns, comparing administration within 0–2 days of life to administration after 3 days of life. Additionally, we aimed to evaluate the associated outcomes.
Methods
Inclusion in this meta-analysis was restricted to studies that met all the following eligibility criteria: (1) randomized trials or non-randomized cohort studies, (2) only preterm infants, (3) same dose of caffeine for both groups, (4) studies available for review in English and in full-text, and (5) studies which reported any of the clinical outcomes of interest. Studies with no control group or placebo as control, different doses of caffeine among intervention and control, other types of xanthines and overlapping study populations were excluded from this analysis.
We systematically searched PubMed, Embase, and Cochrane Central Register of Controlled Trials from inception to March 2023 with the following search: (Neonates OR “preterm infants” OR “preterm neonates” OR infants OR premature OR newborn) AND (caffeine) AND (Early OR timing OR times). Two authors (R.A.P. and T.F.V.B.A.) independently extracted the data following predefined search criteria and quality assessment. If a study reported median and interquartile range, we used methods recommended by Luo et al. [6] and Wan et al. [7] to estimate the mean and standard deviation for data pooling if there were no significant skewness based on the test by Shi et al. [8]. The outcomes included are all-cause mortality, BPD, composite of BPD or all-cause mortality, intraventricular hemorrhage (IVH), necrotizing enterocolitis (NEC), retinopathy of prematurity (ROP), patent ductus arteriosus (PDA), late-onset sepsis, and length of hospital stay (days).
The methodological quality of all included studies was evaluated independently by two authors (C.F.M. and I.M.S.P.) in accordance with the Cochrane Collaboration’s tool. We assessed the risk of bias of randomized controlled trials (RCTs) using the revised Cochrane risk of bias tool for randomized trials (RoB2). The risk of bias of non-randomized comparative studies was assessed using the risk of bias in non-randomized studies of interventions (ROBINS-I) tool. Disagreements were resolved by recruiting a third author to attain consensus [9‒11].
This systematic review and meta-analysis were performed in accordance with the Cochrane Collaboration and the Preferred Reporting Items for Systematic Reviews and Meta-Analysis (PRISMA) statement guidelines [12]. The protocol for this meta-analysis is available in PROSPERO. Odds ratios with 95% confidence intervals were used to compare treatment effects for categorical endpoints and standard difference in mean for continuous variables. Cochran’s Q test and I2 statistics were used to assess for heterogeneity; p values inferior to 0.05 and I2 > 50% were considered significant for heterogeneity. DerSimonian and Laird random-effects models were used [13]. Review Manager 5.4.1 (Cochrane Center, The Cochrane Collaboration, Denmark) was used for statistical analysis.
Results
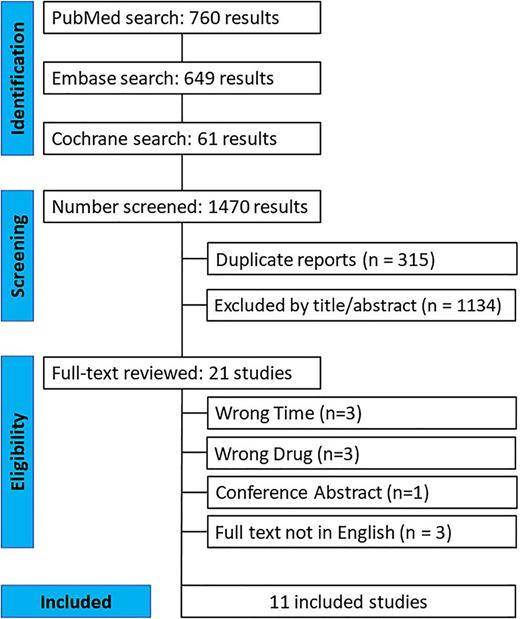
As depicted in Figure 1, the initial search yielded 1,470 results. After removing duplicate records and studies that did not meet the eligibility criteria, 21 studies remained. Following the application of our inclusion and exclusion criteria, a total of 11 studies were included in the final review [3, 14‒23]. Among the patients included in these 11 studies, 78,332 received early caffeine administration, while 44,237 received late caffeine administration. The characteristics of the included studies are presented in Table 1. All studies defined late treatment as initiation of caffeine after 48 h of birth. Early treatment was defined as onset of treatment within 48 h after birth in all studies, except for Ozkan et al. [22] which applied 24 h as the cutoff.
Baseline characteristics of the included studies
| Study . | Population . | Study design . | E/L, N . | Gestational age, weeks . | Birth weight, g . | ||
|---|---|---|---|---|---|---|---|
| . | . | . | . | early . | late . | early . | late . |
| Ozkan et al. [22], 2023 | VLBW (<1,250 g birth weight) | Open-label randomized clinical trial | 45/42 | 27.2±3.2 | 27.8±2.4 | 844±181 | 887±195 |
| Sajjadian et al. [20], 2022 | ≤35 weeks and ≤1,500 g | Open-label randomized clinical trial | 44/46 | 29±3.073 | 29.02±2.985 | 1,048.18±245.23 | 1,108.8±219.049 |
| Yun et al. [14] 2022 | <37 weeks | Retrospective matched cohort | 95/95 | 31.23±2.30 | 30.61±2.14 | 1,530±400 | 1,330±380 |
| Lodha et al. [15], 2015 | <31 weeks | Retrospective cohort | 3,806/1,295 | 28.0 (26.0–29.0) | 28.0 (26.0–30.0) | 1,070 (850–1,310) | 1,050 (790–1,360) |
| Hand et al. [3], 2016 | 23–29 weeks | Retrospective cohort | 85/46 | 26.34±1.84 | 26.41±1.80 | 891.78±227.50 | 909.13±243.92 |
| Dobson et al. [16], 2014 | VLBW (<1,500 g birth weight) | Retrospective cohort (PS) matching | 14,535/14,535 | 28.2 (25.0, 32.0) | 27.7 (24.0, 32.0) | 1,076 (650, 1,450) | 1,009 (560, 1,450) |
| Taha et al. [17], 2014 | VLBW (<1,250 g birth weight) | Retrospective cohort | 1,986/965 | 27.5±2.0 | 27.2±2.1 | 938±201 | 899±216 |
| Patel et al. [18], 2013 | VLBW (<1,250 g birth weight) | Retrospective cohort | 83/57 | 27.3 (25.6–28.7) | 26.6 (25.3–27.7) | 940 (730–1,100) | 910 (715–1,035) |
| Gupte et al. [19], 2016 | ≤32 weeks and <1,500g | Retrospective cohort | 54/67 | 26 (23–31) | 26 (23–30) | 800 (410–1,210) | 822 (520–1,440) |
| Szatkowski et al. [21], 2023 | 22–31 weeks | Retrospective cohort (PS) matching | 13,045/13,045 | 29+1 (27+2–30+5) | 29+3 (27+0–30+6) | 1,170 (895–1,450) | 1,200 (893–1,500) |
| Shenk et al. [23], 2018 | ELBW | Retrospective cohort | 80/58 | 26.4 (25.3–27.4) | 24.9 (24.1–26) | 820 (755–910) | 665 (575–790) |
| Study . | Population . | Study design . | E/L, N . | Gestational age, weeks . | Birth weight, g . | ||
|---|---|---|---|---|---|---|---|
| . | . | . | . | early . | late . | early . | late . |
| Ozkan et al. [22], 2023 | VLBW (<1,250 g birth weight) | Open-label randomized clinical trial | 45/42 | 27.2±3.2 | 27.8±2.4 | 844±181 | 887±195 |
| Sajjadian et al. [20], 2022 | ≤35 weeks and ≤1,500 g | Open-label randomized clinical trial | 44/46 | 29±3.073 | 29.02±2.985 | 1,048.18±245.23 | 1,108.8±219.049 |
| Yun et al. [14] 2022 | <37 weeks | Retrospective matched cohort | 95/95 | 31.23±2.30 | 30.61±2.14 | 1,530±400 | 1,330±380 |
| Lodha et al. [15], 2015 | <31 weeks | Retrospective cohort | 3,806/1,295 | 28.0 (26.0–29.0) | 28.0 (26.0–30.0) | 1,070 (850–1,310) | 1,050 (790–1,360) |
| Hand et al. [3], 2016 | 23–29 weeks | Retrospective cohort | 85/46 | 26.34±1.84 | 26.41±1.80 | 891.78±227.50 | 909.13±243.92 |
| Dobson et al. [16], 2014 | VLBW (<1,500 g birth weight) | Retrospective cohort (PS) matching | 14,535/14,535 | 28.2 (25.0, 32.0) | 27.7 (24.0, 32.0) | 1,076 (650, 1,450) | 1,009 (560, 1,450) |
| Taha et al. [17], 2014 | VLBW (<1,250 g birth weight) | Retrospective cohort | 1,986/965 | 27.5±2.0 | 27.2±2.1 | 938±201 | 899±216 |
| Patel et al. [18], 2013 | VLBW (<1,250 g birth weight) | Retrospective cohort | 83/57 | 27.3 (25.6–28.7) | 26.6 (25.3–27.7) | 940 (730–1,100) | 910 (715–1,035) |
| Gupte et al. [19], 2016 | ≤32 weeks and <1,500g | Retrospective cohort | 54/67 | 26 (23–31) | 26 (23–30) | 800 (410–1,210) | 822 (520–1,440) |
| Szatkowski et al. [21], 2023 | 22–31 weeks | Retrospective cohort (PS) matching | 13,045/13,045 | 29+1 (27+2–30+5) | 29+3 (27+0–30+6) | 1,170 (895–1,450) | 1,200 (893–1,500) |
| Shenk et al. [23], 2018 | ELBW | Retrospective cohort | 80/58 | 26.4 (25.3–27.4) | 24.9 (24.1–26) | 820 (755–910) | 665 (575–790) |
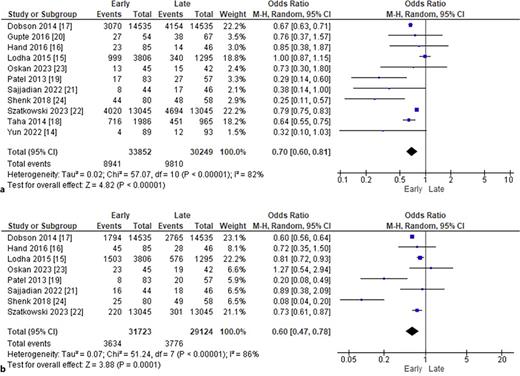
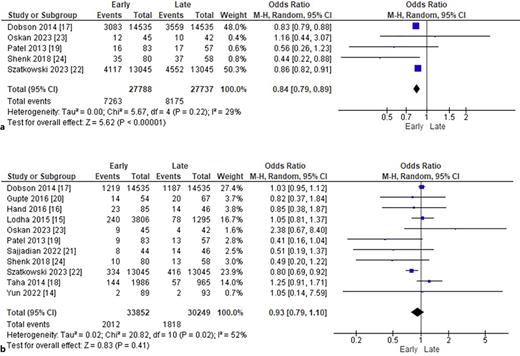
In the group receiving early caffeine administration, there was a significant decrease in the rates of BPD (OR: 0.70; 95% CI: 0.60–0.81; p < 0.00001; I2 = 82%; Fig. 2a), PDA (OR: 0.60; 95% CI: 0.47–0.78; p = 0.00001; I2 = 86%; Fig. 2b), IVH (OR: 0.86; 95% CI: 0.82–0.90; p < 0.00001; I2 = 0%; Fig. 3a), ROP (OR: 0.80; 95% CI: 0.74–0.86; p < 0.00001; I2 = 20%; Fig. 3b), and late-onset sepsis (OR: 0.84; 95% CI: 0.79–0.89; p < 0.00001; I2 = 29%; Fig. 4a). There was no statistically significant difference between early versus late caffeine administration for the outcome of NEC (OR: 0.93; 95% CI: 0.79–1.10; p = 0.41; I2 = 52%; Fig. 4b).
a Comparison of BPD rates by timing of caffeine administration. b Comparison of PDA rates by timing of caffeine administration.
a Comparison of BPD rates by timing of caffeine administration. b Comparison of PDA rates by timing of caffeine administration.
a Comparison of IVH rates by timing of caffeine administration. b Comparison of ROP rates by timing of caffeine administration.
a Comparison of IVH rates by timing of caffeine administration. b Comparison of ROP rates by timing of caffeine administration.
a Comparison of late-onset sepsis rates by timing of caffeine administration. b Comparison of NEC rates by timing of caffeine administration.
a Comparison of late-onset sepsis rates by timing of caffeine administration. b Comparison of NEC rates by timing of caffeine administration.
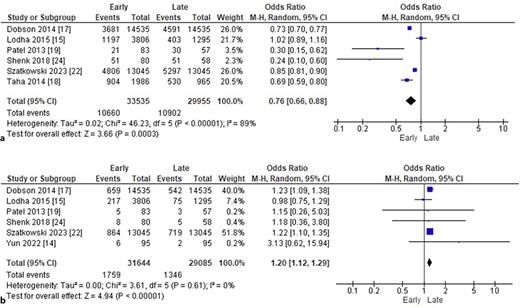
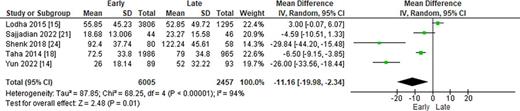
The incidence of the composite outcome of BPD or death was significantly lower in the early group (OR: 0.76; 95% CI: 0.66–0.88; p = 0.0003; I2 = 89%; Fig. 5a). However, early caffeine administration was associated with a higher rate of mortality as compared with late administration (OR: 1.20; 95% CI: 1.12–1.29; p < 0.00001; I2 = 0%; Fig. 5b). Duration of hospital stay was significantly reduced in the early caffeine administration group, with a pooled mean difference of −11.2 days (95% CI: −20.0 to −2.3 days; p = 0.01; I2 = 94; Fig. 6).
a Comparison of BPD or death rates by timing of caffeine administration. b Comparison of death rates by timing of caffeine administration.
a Comparison of BPD or death rates by timing of caffeine administration. b Comparison of death rates by timing of caffeine administration.
Comparison of length of hospital stay (days) by timing of caffeine administration.
Comparison of length of hospital stay (days) by timing of caffeine administration.
Risk of Bias Assessment
The methodological quality of the randomized studies was assessed using the RoB 2.0 tool [10]. Reviewers independently evaluated the risk of bias across various domains, including randomization, allocation to intervention, adherence to intervention, handling of missing outcome data, measurement of outcome, and selection of reported results. The overall risk of bias was categorized as “low,” “some concerns,” or “high” for each domain, both across studies and within individual studies. All randomized studies were rated as having a low risk of bias (Table 2).
Domain-specific risk of bias and overall assessment for included randomized studies using RoB 2 tool
| Study . | Bias from randomization process . | Bias due to deviations from intended interventions . | Bias due to missing outcome data . | Bias in measurement of the outcomes . | Bias in selection of the reported result . | Overall risk of bias . |
|---|---|---|---|---|---|---|
| Ozkan et al. [22], 2023 | Low | Low | Low | Low | Low | Low |
| Sajjadian et al. [20], 2022 | Low | Low | Low | Low | Low | Low |
| Study . | Bias from randomization process . | Bias due to deviations from intended interventions . | Bias due to missing outcome data . | Bias in measurement of the outcomes . | Bias in selection of the reported result . | Overall risk of bias . |
|---|---|---|---|---|---|---|
| Ozkan et al. [22], 2023 | Low | Low | Low | Low | Low | Low |
| Sajjadian et al. [20], 2022 | Low | Low | Low | Low | Low | Low |
Most studies included were observational and were evaluated using the ROBINS-I tool [11], which categorizes studies based on seven domains: confounding, selection of participants, classification of interventions, deviations from intended interventions, missing data, measurement of outcomes, and selection of reported results. Differences between groups, such as higher birth weight or the use of estrogen during pregnancy, contributed to a moderate risk of bias in the second domain. Missing data also increased the risk in retrospective analyses. Despite these limitations, the correct statistical analysis and minimal impact on the outcome allowed a “moderate risk of bias” for these studies. This means that they provide solid evidence for non-randomized studies but cannot be considered equivalent to well-conducted randomized trials. Overall, they were judged to be at a low risk of bias in most domains (Table 3).
Domain-specific risk of bias and overall assessment for included non-randomized studies using ROBINS-I tool
| Study . | Bias due to confounding . | Bias in selection of participants . | Bias in classification of interventions . | Bias due to deviations from intended interventions . | Bias due to missing data . | Bias in measurement of outcomes . | Bias in selection of the reported result . | Overall risk of bias judgment . |
|---|---|---|---|---|---|---|---|---|
| Szatkowski et al. [21], 2023 | Moderate | Low | Low | Low | Low | Low | Low | Moderate |
| Yun et al. [14], 2022 | Moderate | Low | Low | Low | Low | Low | Low | Moderate |
| Shenk et al. [23], 2018 | Moderate | Low | Low | Low | Low | Low | Low | Moderate |
| Gupte et al. [19], 2016 | Moderate | Low | Low | Moderate | Moderate | Low | Low | Moderate |
| Hand et al. [3], 2016 | Moderate | Ni/moderate risk | Low | Low | Low | Low | Low | Moderate |
| Lodha et al. [15], 2015 | Moderate | Low | Low | Low | Low | Low | Low | Moderate |
| Taha et al. [17], 2014 | Moderate | Low | Low | Moderate | Low | Low | Low | Moderate |
| Dobson et al. [16], 2014 | Moderate | Low | Low | Low | Low | Low | Low | Moderate |
| Patel et al. [18], 2013 | Moderate | Moderate | Low | Low | Low | Low | Low | Moderate |
| Study . | Bias due to confounding . | Bias in selection of participants . | Bias in classification of interventions . | Bias due to deviations from intended interventions . | Bias due to missing data . | Bias in measurement of outcomes . | Bias in selection of the reported result . | Overall risk of bias judgment . |
|---|---|---|---|---|---|---|---|---|
| Szatkowski et al. [21], 2023 | Moderate | Low | Low | Low | Low | Low | Low | Moderate |
| Yun et al. [14], 2022 | Moderate | Low | Low | Low | Low | Low | Low | Moderate |
| Shenk et al. [23], 2018 | Moderate | Low | Low | Low | Low | Low | Low | Moderate |
| Gupte et al. [19], 2016 | Moderate | Low | Low | Moderate | Moderate | Low | Low | Moderate |
| Hand et al. [3], 2016 | Moderate | Ni/moderate risk | Low | Low | Low | Low | Low | Moderate |
| Lodha et al. [15], 2015 | Moderate | Low | Low | Low | Low | Low | Low | Moderate |
| Taha et al. [17], 2014 | Moderate | Low | Low | Moderate | Low | Low | Low | Moderate |
| Dobson et al. [16], 2014 | Moderate | Low | Low | Low | Low | Low | Low | Moderate |
| Patel et al. [18], 2013 | Moderate | Moderate | Low | Low | Low | Low | Low | Moderate |
Discussion
In this systematic review and meta-analysis, we utilized observational studies and RCTs to compare the benefits and complications associated with early administration of caffeine within the first 3 days of life versus administration of caffeine after 3 days of life. Although the outcomes mentioned were previously demonstrated in the earlier meta-analyses conducted by Kua et al. [4] and Park et al. [5], the present study possesses unique characteristics that enhance the evidence and support these conclusions. First, there was a limited number of studies available at the time. Kua et al. [4] performed their meta-analysis, with only four studies included and an overall patient population size that was less than a half of the size of our study. Furthermore, the most recent meta-analysis on this topic conducted by Park et al. [5] also had a patient population size that was less than a half of our study. Second, both previous meta-analyses assessed the risk of bias using the Newcastle-Ottawa Scale, which is no longer recommended in current practice.
In preterm infants, BPD has a high incidence and is considered one of the leading causes of neonatal morbidity and mortality. Various pharmacotherapies, including dexamethasone, hydrocortisone, vitamin A, and macrolides, are being investigated as potential preventive therapies, and neither of them could have expressive results in the prevention setting [24, 25]. Although most of these studies are observational, the use of caffeine has consistently demonstrated significant benefits, including improved respiratory function, enhanced pulmonary compliance, increased respiratory muscle contractility, reduced airway resistance, and earlier discontinuation of mechanical ventilation [26‒29]. Our study confirms these benefits with statistical significance.
In the Caffeine for Apnea of Prematurity Trial (CAP trial) [1], there was no significant difference in mortality between the caffeine group and the non-caffeine group. Otherwise, in studies conducted by Dobson et al. [16], Patel et al. [18], Shenk et al. [23], Szatkowski et al. [21], and Yun et al. [14] as well as in this current meta-analysis, there was a significantly higher rate of mortality in the early caffeine group. Given the improvement in other outcomes, these results may potentially be due to survival bias favoring the late caffeine initiation group. Due to increased mortality in this population in the first 24 h of life, a substantial proportion of preterm infants may not survive to be included in the late caffeine initiation group. Therefore, these infants with a worse prognosis may be included preferentially in the early treatment group.
Necrotizing enterocolitis is recognized as one of the major emergent conditions during the neonatal period. The prevailing theory suggests that NEC arises from ischemic necrosis of the intestinal mucosa, which is closely associated with severe inflammation [30, 31]. Although the exact cause of ischemia is not well understood, its incidence is particularly linked to preterm infants [32, 33]. Caffeine has been investigated as a potential substance to decrease the occurrence of NEC. In the context of NEC, studies by Lodha et al. [15], Ozkan et al. [22], and Taha et al. [17] support the administration of caffeine at a later stage. The CAP trial [1] demonstrated no significant effect of caffeine initiated within the first 10 days after birth on NEC incidence. Similarly, this meta-analysis did not identify any statistically significant difference in the incidence of NEC between the two groups.
Clinical studies have demonstrated that caffeine possesses neuroprotective effects in premature infants, including mitigating hypoxia-induced white matter damage, enhancing ventilation function, and promoting brain self-regulation [34]. While two studies conducted by Ozkan et al. [22] and Patel et al. [18] supported late caffeine administration, our study revealed that early caffeine administration yielded significantly better outcomes in terms of IVH.
ROP is another complication frequently observed in preterm neonates. The progression of ROP is associated with hypoxia, which triggers the formation of free radicals and subsequently increases the permeability of retinal vessels, leading to edema and hemorrhage [35‒39]. Among the studies included in this comparison, only the study conducted by Hand et al. [3] reported benefits of late caffeine administration for ROP prevention. Conversely, our study demonstrated statistical significance supporting the early use of caffeine in the prevention of ROP.
Regarding patent ductus arteriosus, among the studies included in our analysis, only the study conducted by Ozkan et al. [22] reported a more favorable outcome with late caffeine administration. The association between caffeine therapy and a decreased incidence of PDA requiring treatment is further supported by various cardiac mechanisms, including improvements in cardiac function, modulation of fluid balance, and effects on ductal constriction, specifically by increasing the concentration of cyclic adenosine monophosphate. Caffeine has been shown to increase cardiac output and blood pressure in both preterm infants and adults. Additionally, a reduced need for PDA ligation was observed in the Caffeine for Apnea of Prematurity Trial [1].
This meta-analysis faced some limitations. There were only two RCTs out of the 11 included studies. Therefore, our meta-analysis may be subject to confounding. Nevertheless, we included propensity score matched data for all outcomes, when available. Unfortunately, this does not eliminate the risk of selection bias, including the potential for survival bias favoring a higher mortality in the early caffeine initiation group, as previously discussed. There was also some variability in the definition of outcomes between different studies or, alternatively, absence of outcome definition in some of the included studies. Whether this could have influenced outcomes is unclear, but it is unlikely to favor one group versus another.
Conclusions
This systematic review and meta-analysis demonstrated an association of early caffeine administration with a reduction of BPD, IVH, ROP, late-onset sepsis, and PDA in preterm neonates, as compared with late caffeine initiation. However, there was also evidence of increased mortality in the early caffeine administration group. Although the latter could potentially be explained by survival bias in predominantly observational studies, additional RCTs of early versus late caffeine administration in this patient population are warranted prior to a definitive recommendation for early caffeine initiation.
Statement of Ethics
An ethics statement is not applicable because this study is based exclusively on published literature.
Conflict of Interest Statement
All authors report no relationships that could be construed as a conflict of interest. All authors take responsibility for all aspects of the reliability and freedom from bias of the data presented and their discussed interpretation.
Funding Sources
There were no funding sources.
Author Contributions
V.K.V. tested the viability, wrote the search and inclusion criteria, and registered this meta-analysis in PROSPERO. T.F.V.B.A. and R.A.P. conducted deduplication and article screening and were also in charge of statistical analysis. V.K.V., I.M.S.P., and C.F.M. were responsible for data and outcome collection. I.M.S.P. and C.F.M. assessed the risk of bias. All authors contributed to the abstract and the manuscript writing.
Data Availability Statement
Authors confirm that the data that support the findings of this study are available within the article. Further inquiries can be directed to the corresponding author.